The Policies, Procedures, Guidance, and Documents that are part of the Human Research Protection Program at Harvard University Area IRB are collectively known as the “Toolkit”. The Toolkit consists of “living” documents that will be updated as our regulatory landscape changes. We will also provide additional guidance and IRB decision aids to assist the research community through the IRB review process. Here is an overview of the components of the Toolkit:
Human Research Protection Program (HRPP) Plan: The HRPP Plan provides an overview of the Human Research Protection Program at Harvard University. The document defines roles, mission, and includes a complete outline of the regulatory requirements that must be adhered to. It is the "30,000 foot view" document that ties all of the pieces of our program together.
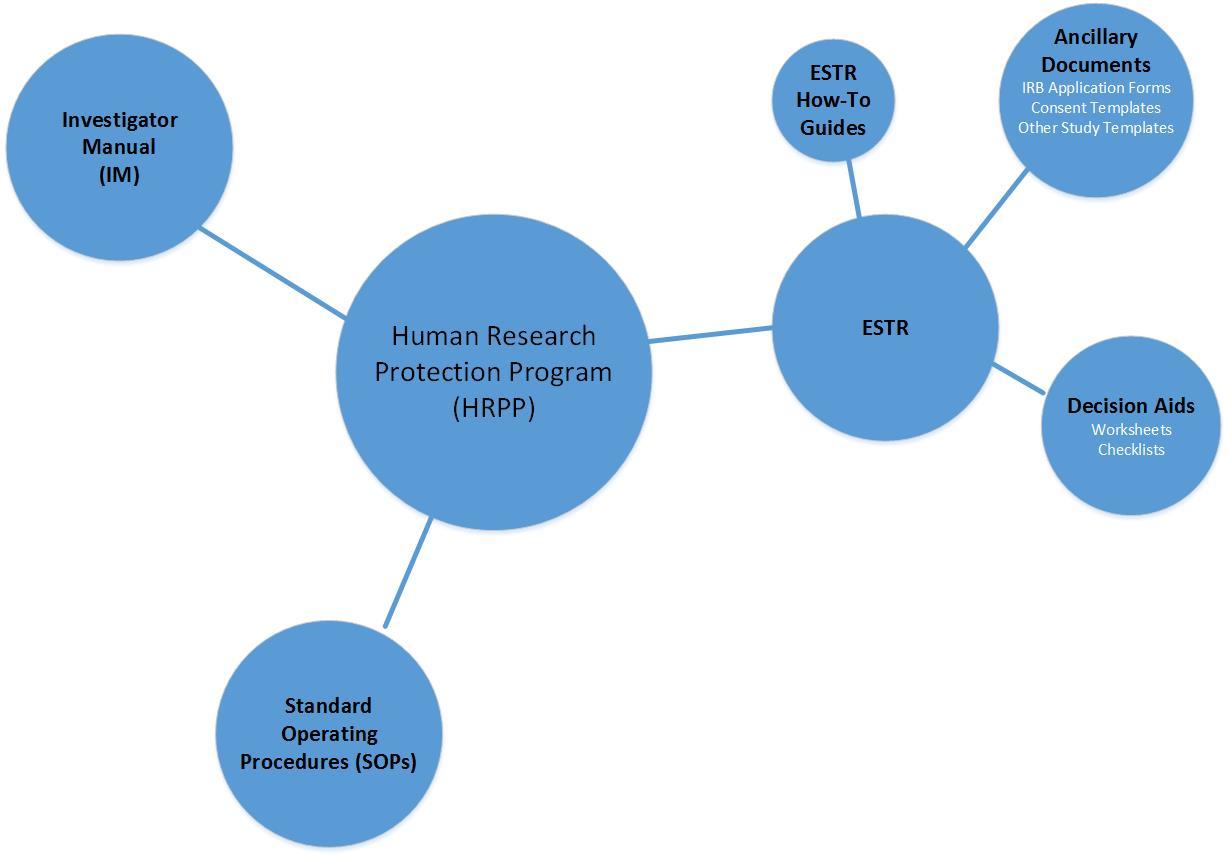
Investigator Manual (IM): The IM provides additional guidance and information on various regulatory topics, insight into nebulous areas of IRB review, and resources that assist in navigating the IRB process.
Standard Operating Procedures (SOPs): SOPs act as our “instruction manual.” They are comprised of the “nuts and bolts” of the IRB review process in our electronic submission system (ESTR) and policy that guides the human subject research review process.
Electronic Submission, Tracking, and Reporting (ESTR): ESTR is the electronic submission system that automates the IRB review process for the Harvard IRBs (Harvard University Area and Longwood Medical Area).
These guides provide step-by-step instructions for the ESTR system according to the user profile:
- Study Submission Guide: for Principal Investigators and study staff
- Ancillary Reviewer Guide: for faculty sponsor, department or other non-IRB ancillary reviewers
- Study Reviewer's Guide: for IRB reviewers
- IRB Staff Administration Guide: for IRB staff
ESTR Decision Aids
The ESTR system library (you must be logged into ESTR) contains resourceful worksheets and checklists.
- Checklists are completed by IRB staff to document regulatory decisions according to the human subject protection regulations. Worksheets can also be helpful for researchers to gain insight into the regulatory determinations that are being made.
- Worksheets are used by IRB staff and researchers to assist in regulatory decisions. IRB staff may also request that specific worksheets be completed by researchers to gather information for a particular regulatory determination.
Ancillary Documents
The ESTR system library and IRB website list the forms and templates that are needed to complete an IRB submission. Ancillary documents are available in the ESTR system and provide needed regulatory information for a study.
- IRB Submission Forms: The CUHS Protocol Template is the main application form that is used to submit all IRB submissions with the exception of a Not Human Subjects Research Determination as there is a separate form for that type of submission. Both forms have been revised to gather important information and reduce the back and forth that occurs from not having all the required information at the outset.
- Supplemental Forms: These forms include the Financial Interest Disclosure Form when there is a financial interest to disclose as well forms to complete when including non-Harvard personnel in a study or when there is a request for a reliance agreement between IRBs. The ESTR system contains questions that let researchers know when one or more of these forms is needed. If one is missed, don’t worry – IRB staff will let you know.
- Consent Form Templates: The regulations require that certain elements are included in consent forms that are provided to subjects in research. There are also specific elements required if a research study includes sharing of genetic data or if there is a Certificate of Confidentiality, to name just a few. The consent form templates include all required elements that may needed. If a certain element does not apply, then just delete that section from the template form.

